Abstract
Background: Diffuse large B cell lymphoma with double expression of myc/BCL2(DE-DLBCL), is usually responsive poorly to standard therapy of R-CHOP regimen, And the long-term survival rate of patients with DE-DLBCL is only 30% - 40%. Recently, Phoenix study demonstrate Ibrutinib plus R-CHOP improved EFS and OS (versus placebo plus R-CHOP) for DE-DLBCL younger patients. While for elder patients, the outcome makes no difference between two groups, due partly to the poor tolerance in Ibrutinib group. The novel BTK inhibitor, zanubrutinib, have better safety profile. So we suppose zanubrutinib plus R-CHOP (ZR-CHOP) may improve the outcome of DE-DLBCL even in older patients.
Objective: To investigate the response rate, PFS (progression free survival) and safety of RCHOP regimen combined with zanubrutinib for the first-line treatment of DE-DLBCL patients.
Method: We initiate a prospective, single arm, phase II clinical trial. Standard dose of Zanubrutinib plus R-CHOP regimen (ZR-CHOP) was adopted in our center as first-line treatment for patients(18-75yrs) with DE-DLBCL. DE-DLBCL is defined as ≧40% positive rate for myc and ≧50% for bcl2 by immunohistochemistry, and double hit cases were excluded through FISH test (myc, bcl2 and bcl6). The patients in Ann Arbor stage I were excluded. The standard 6 -8cycles of ZR-CHOP plus 2R were administered as induction therapy, with no maintenance therapy. Two-year-PFS was the primary endpoint. PET-CT after 4 cycles and 6 cycles were used for assessment of response, but CT scanning was used after 2 cycles of therapy for primary assessment.
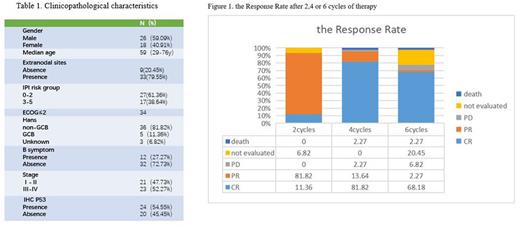
Results: From November 6, 2020 to July 20, 2022, 46 patients with DE DLBCL were enrolled,two of them voluntarily quit. And 44 patients completed 4 cycles of chemotherapy,36 patients completed 6 cycles of chemotherapy. The baseline characteristics of the patients are listed in Table 1. After 2 cycles of therapy, 5/44 patients achieved CR (11.36%), 36/44 PR (81.82%) and 3/44 not evaluated(6.82%). After 4 cycles of therapy, 36/44 got CR (81.82%), 6/44 PR (13.64%) ,1/44 PD (2.27%), and 1/34 died due to cardiovascular events (2.27%). After 6 cycles of therapy, 30/44 CR (68.18%), 1/44 PR (2.27%) , 3/44 PD (6.82%),and 1/44 died due to cardiovascular events(2.27%) and 9/44 not evaluated(20.45%)(Figure 1).In addition, one patient relapsed 3 months after chemotherapy. The response rate after 6 cycles of ZRCHOP (36 patients) regimen was obviously higher than that of the previous report after R-CHOP regimen for DE-DLBCL (86.11%VS 70%). After median follow-up of 9 month(2-20m), the PFS rate at 6 month is 97.4%(CI 94.8-100), the PFS rate at 12month is 85%(CI 77.8-92.2).Totally five patients progressed during or shortly after the induction therapy. Five patients had molecular genetic high-risk factors, including TP53 mutation, ATM mutation, P53 protein expression, and CD5 protein expression. Among of the five patients are still alive four patients after second line therapy including auto-stem cell transplantation, one patient were died after CAR-T therapy.
About the safety of the study, grade 3 or more severe AEs were recorded are slightly less than The PHOENIX study. No adverse cardiovascular reactions of any level were found in this study.
Conclusions: Despite of the small sample size and short follow-up time, ZRCHOP regimen achieved very high CR rate for DE DLBCL, and the toxicities were acceptable. Further study are required to prove the benefit of zanubrutinib when added to R-CHOP for newly-diagnosed DE-DLBCL. TP53 mutation , ATM mutation,P53 protein expression, and CD5 protein expression were probably the main reason for disease progression.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
To further improve the survival benefits,we design the Zanubrutinib PLUS RCHOP regimen to achieves high complete response rate in the treatment of newly-diagnosed double-expression diffuse large B cell lymphoma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal